How does tFUS work?
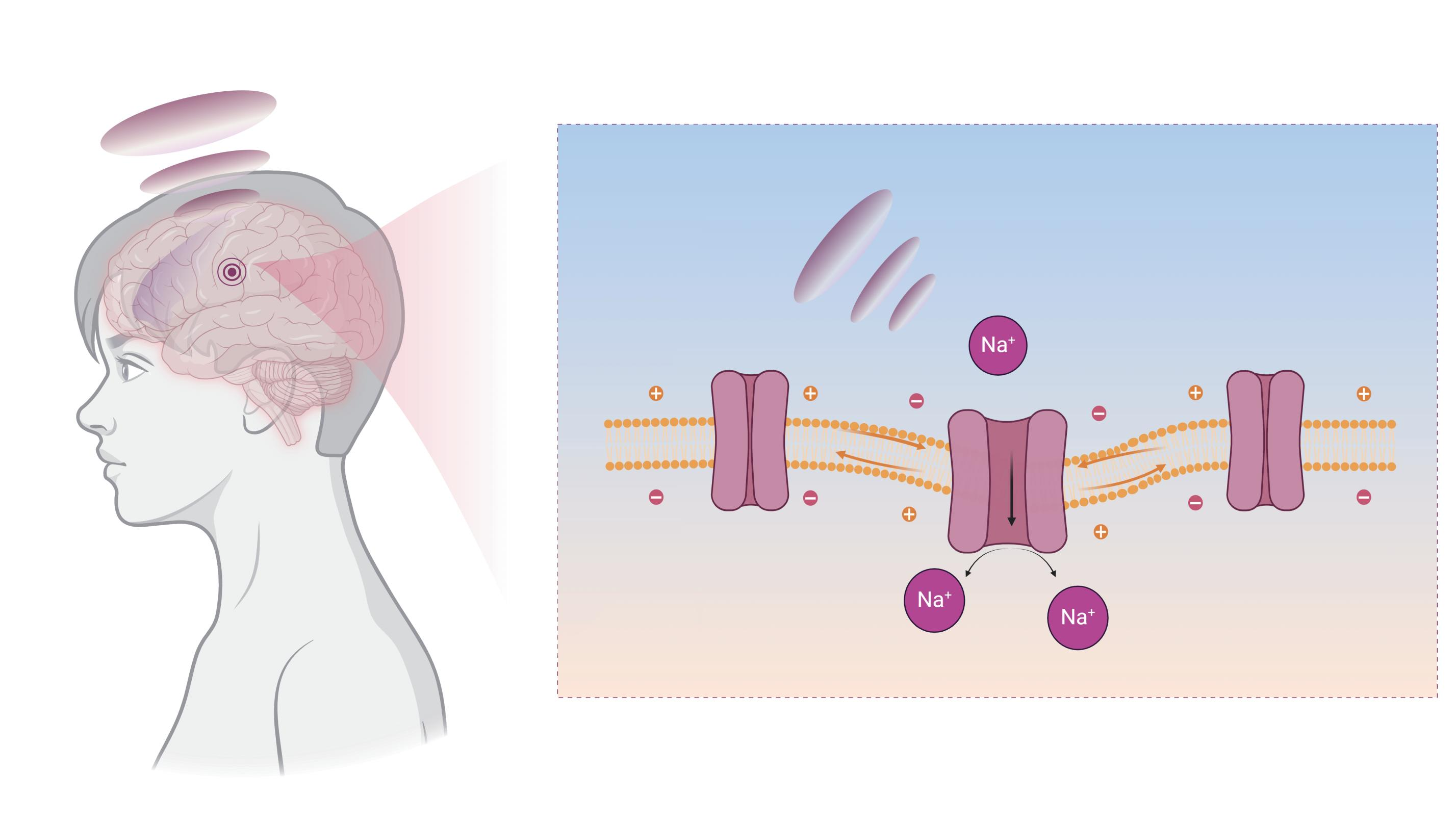
Low Intensity Transcranial Focused Ultrasound Stimulation (tFUS) is an emerging, non-invasive physical nerve rehabilitation technique that does not cause tissue damage. The principle of neural regulation of sound radiation force is mainly that the ultrasound transducer placed on the scalp focuses rhythmically low-intensity ultrasound pulses on the target brain tissue. The mechanosensitive ion channels on the neurons in the target area sense the sound radiation force and transition from a closed state to an open state, inducing ion exchange inside and outside the membrane, thereby regulating the generation and transmission of action potentials, achieving the purpose of exciting or inhibiting brain functional activity.
What population can tFUS be used for?
- Psychiatry: depression, mania, schizophrenia, bipolar disorder, obsessive-compulsive disorder, sleep disorders, etc;
- Neurology: epilepsy, Parkinson's disease, Alzheimer's disease, neurogenic tinnitus, neuropathic pain, etc;
- Rehabilitation department: stroke, spinal cord injury, cerebral palsy, chronic pain, virtual injury, etc;
- Pediatrics: cerebral palsy, multiple seizures, autism, autism, attention deficit hyperactivity disorder, etc;
- Other: cognitive impairment recovery, swallowing disorders, addiction, visual neglect, brain damage, etc.
How should the treatment course of tFUS be set up?
Due to the differences in the mechanisms of tFUS in treating different diseases and the heterogeneity of patients' responses to tFUS treatment, the course of treatment for different diseases with tFUS also varies. Taking depression as an example, it is generally believed that 1-4 weeks is the initial acute treatment, and 5-12 weeks is the later consolidation treatment. Other indications can be adjusted according to the actual needs of this course of treatment.
Is there a conflict between tFUS treatment and medication treatment?
Do tFUS treatments have any side effects?
How secure is tFUS?
In the past 20 years, the accumulation of a large number of basic research results has laid a solid technological foundation for their application to the human body. Based on this background, the first achievement of ultrasound in human neural regulation was published in 2013. Professor Hameroff from the Health Sciences Center at the University of Arizona has applied ultrasound to the frontal lobe of pain patients for the first time, opening the curtain on non-invasive ultrasound neural regulation in humans. Currently, there are nearly 70 high-quality clinical reports on the application of ultrasound in human brain neural regulation, including healthy individuals, chronic pain, consciousness disorders, Alzheimer's disease, Parkinson's disease, depression, schizophrenia, anxiety disorders, medication use disorders, epilepsy, stroke and other diseases, with more than 1000 participants. In 2022, the team led by Sarica et al. from Canada included 35 articles based on Web of Science and PubMed databases, with a total of 667 participants. The analysis results showed that out of 667 participants, a total of 14 experienced discomfort symptoms, with a probability of inducing adverse reactions of 3.4% (14/677). Possible adverse reactions include headache, scalp fever, neck pain, anxiety, drowsiness, etc. Adverse reactions that occur are mild, brief, and mostly self relieving or disappear in a short period of time. In 2024, Lee et al. from South Korea retrieved 64 research reports on human central nervous system ultrasound neural regulation published between 2013 and 2023, involving over 800 subjects. After analysis, similar conclusions were drawn, namely: ① Adverse reactions are brief and mild, generally self relieving or subsiding within 24 hours; ② The longest duration of adverse reactions was 3 weeks, with transient memory abnormalities observed in one drug-resistant epilepsy patient No serious adverse events; ④ It is still unclear whether the adverse reactions observed are directly related to ultrasound. Therefore, tFUS is a non-invasive and safe neural regulation technology.








